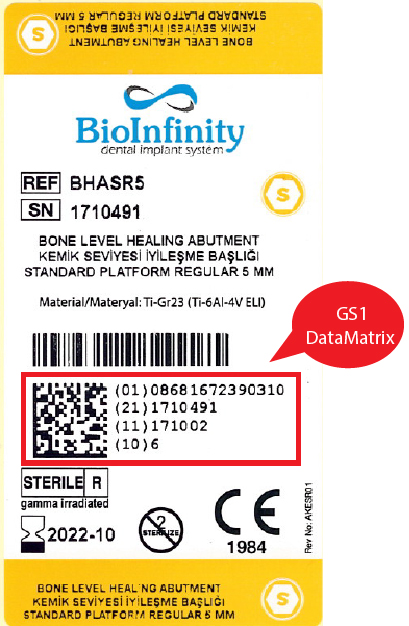
The Unique Device Identification (UDI) is a system used to mark and identify medical devices within the healthcare supply chain.
The IMDRF (International Medical Device Regulator Forum), the United States Food and Drug Administration (FDA) and the European Commission are aiming for a globally harmonized and consistent approach to increase patient safety and help optimize patient care by proposing a harmonized legislation for Unique Device Identification (UDI), using global standards.
The U.S. FDA released, in September 2013 a rule which establishes that a common, worldwide system for product identification should be applied to all medical devices placed on the U.S. market. The rule establishes that:
On 17 December 2013, GS1 has been accredited by the U.S. Food and Drug Administration (FDA) as issuing agency for unique device identifiers (UDIs).